![The pH scale is a scale that expresses the hydronium ion concentration, [H 3 O + ], in an aqueous solution using log base 10. The pH scale is a scale that expresses the hydronium ion concentration, [H 3 O + ], in an aqueous solution using log base 10.](http://ch302.cm.utexas.edu/images302/phScale.jpg)
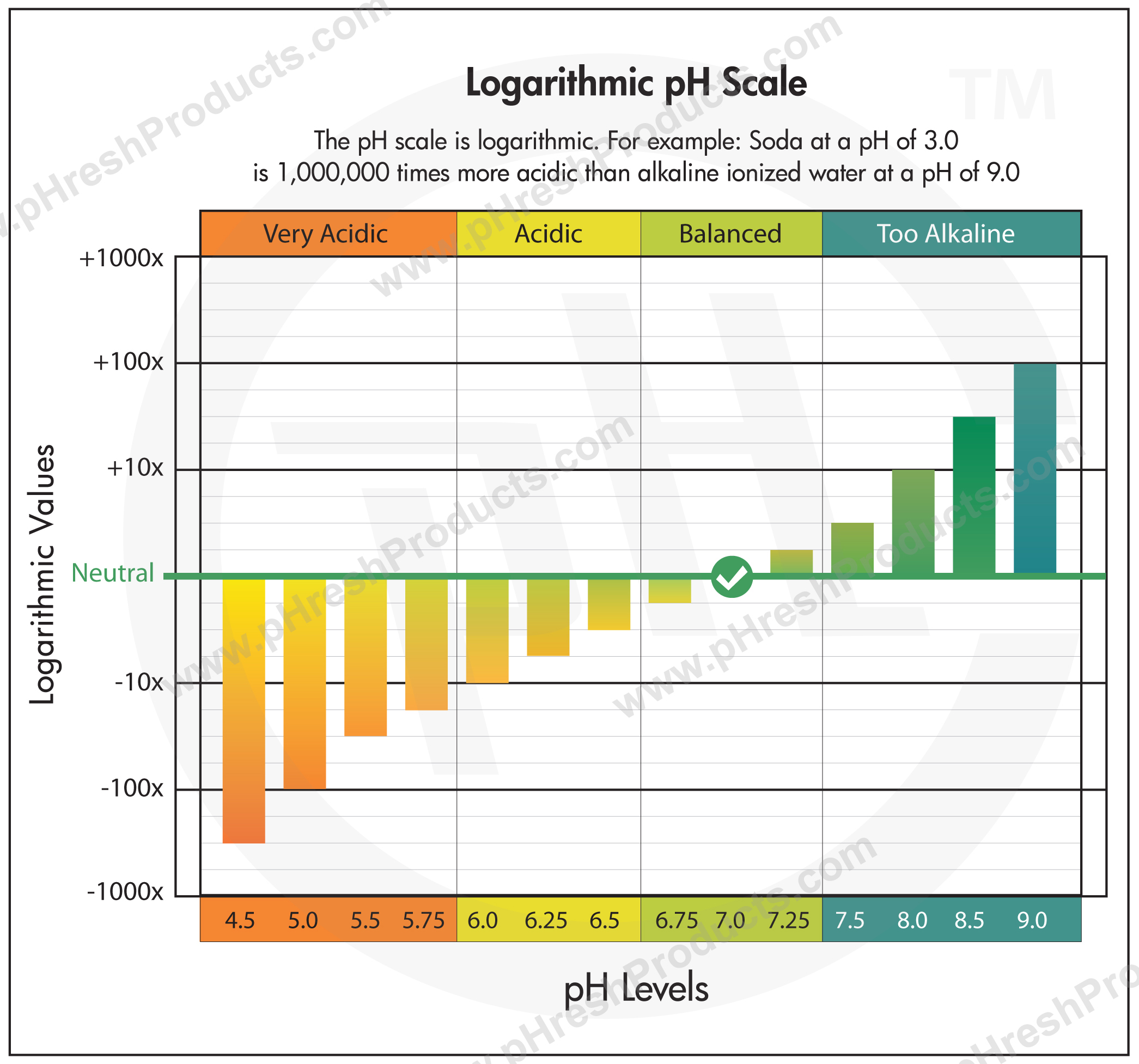
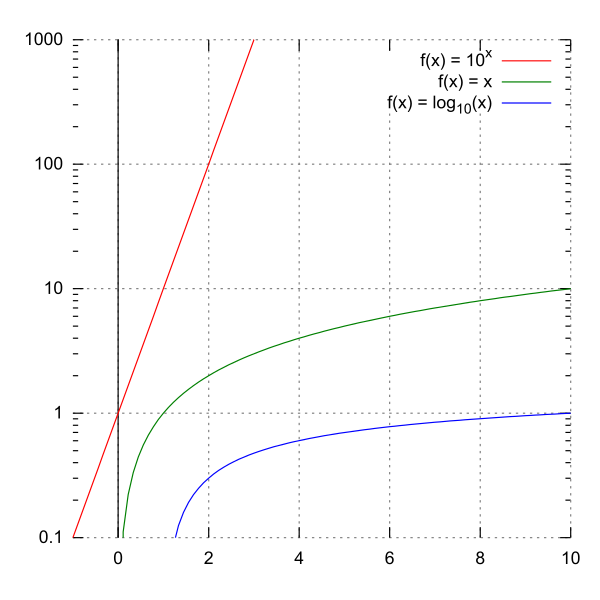
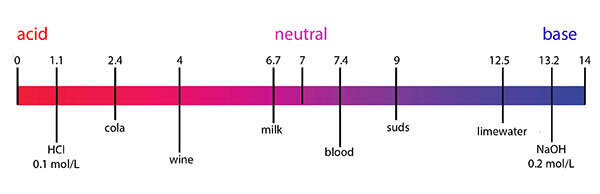
The pH scale is a scale that expresses the hydronium ion concentration, [H 3 O + ], in an aqueous solution using log base 10.
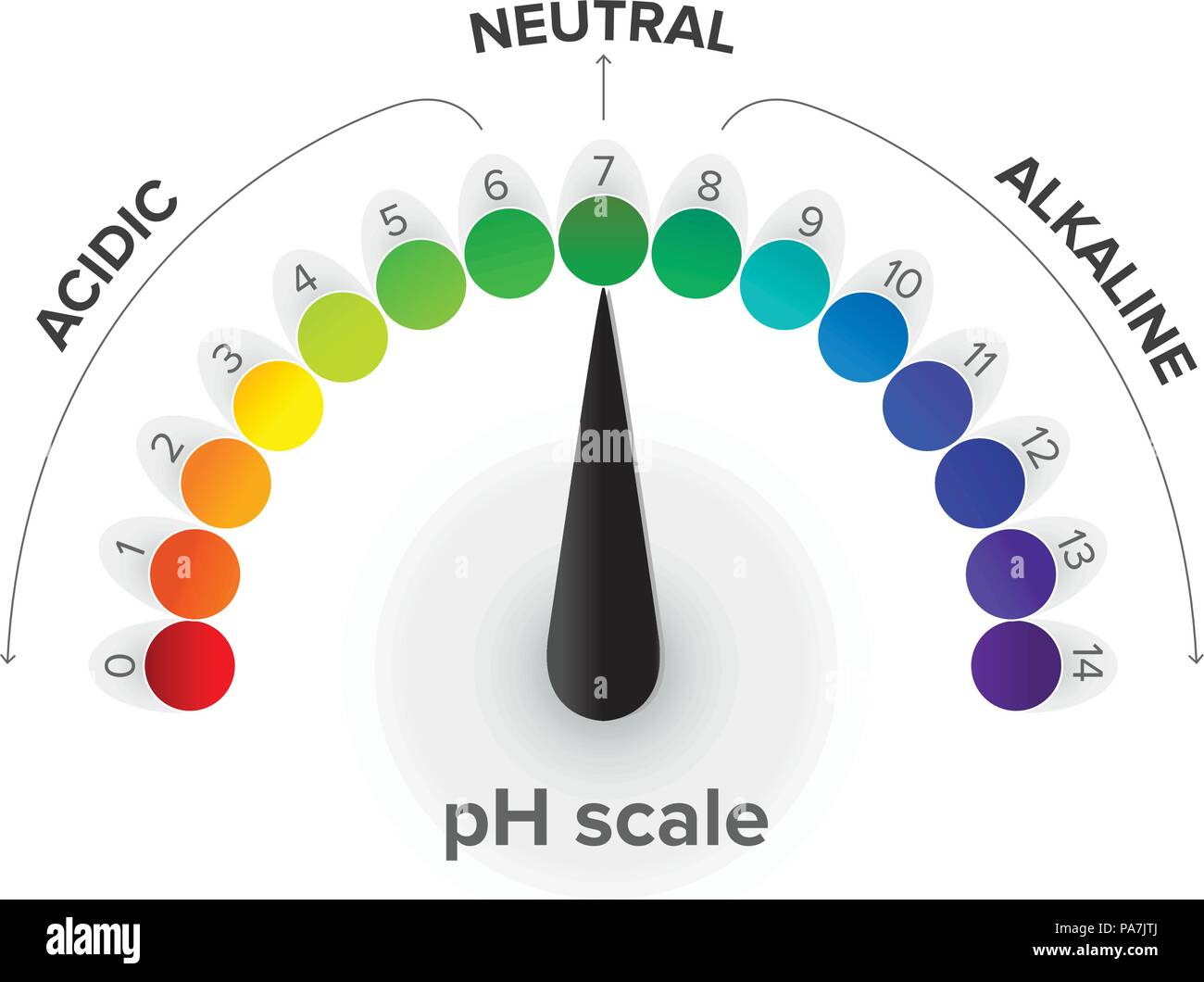
Measurement of the pH scale, pressure gauge, infographics. pH is a logarithmic scale used to specify the acidity or basicity of an aqueous solution Stock Vector Image & Art - Alamy
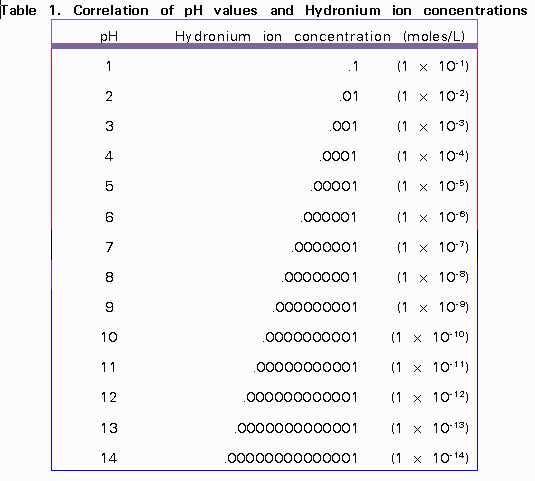
![SOLVED: 1. pH is a logarithmic scale. This means that for a change of pH 3 to pH 2, the hydronium ion concentration [H3O+] changes by a factor of? 2. Acids have SOLVED: 1. pH is a logarithmic scale. This means that for a change of pH 3 to pH 2, the hydronium ion concentration [H3O+] changes by a factor of? 2. Acids have](https://cdn.numerade.com/ask_previews/48330dbe-2875-4cae-b450-b3566371f267_large.jpg)
SOLVED: 1. pH is a logarithmic scale. This means that for a change of pH 3 to pH 2, the hydronium ion concentration [H3O+] changes by a factor of? 2. Acids have
![pH scale Logarithmic scale expressing the H + concentration, [H + ]. If the pH changes by a factor of 1, the [H + ] changes by a factor of 10. pH = - ppt download pH scale Logarithmic scale expressing the H + concentration, [H + ]. If the pH changes by a factor of 1, the [H + ] changes by a factor of 10. pH = - ppt download](https://images.slideplayer.com/22/6410049/slides/slide_2.jpg)
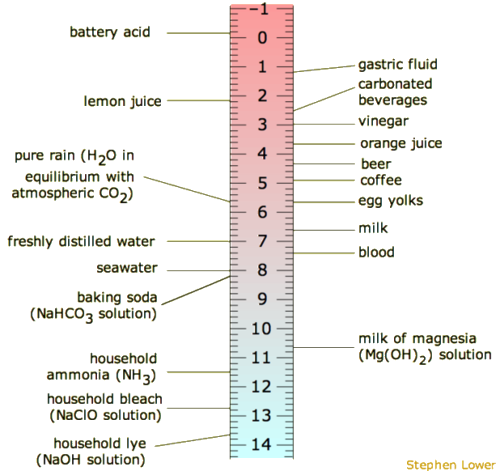
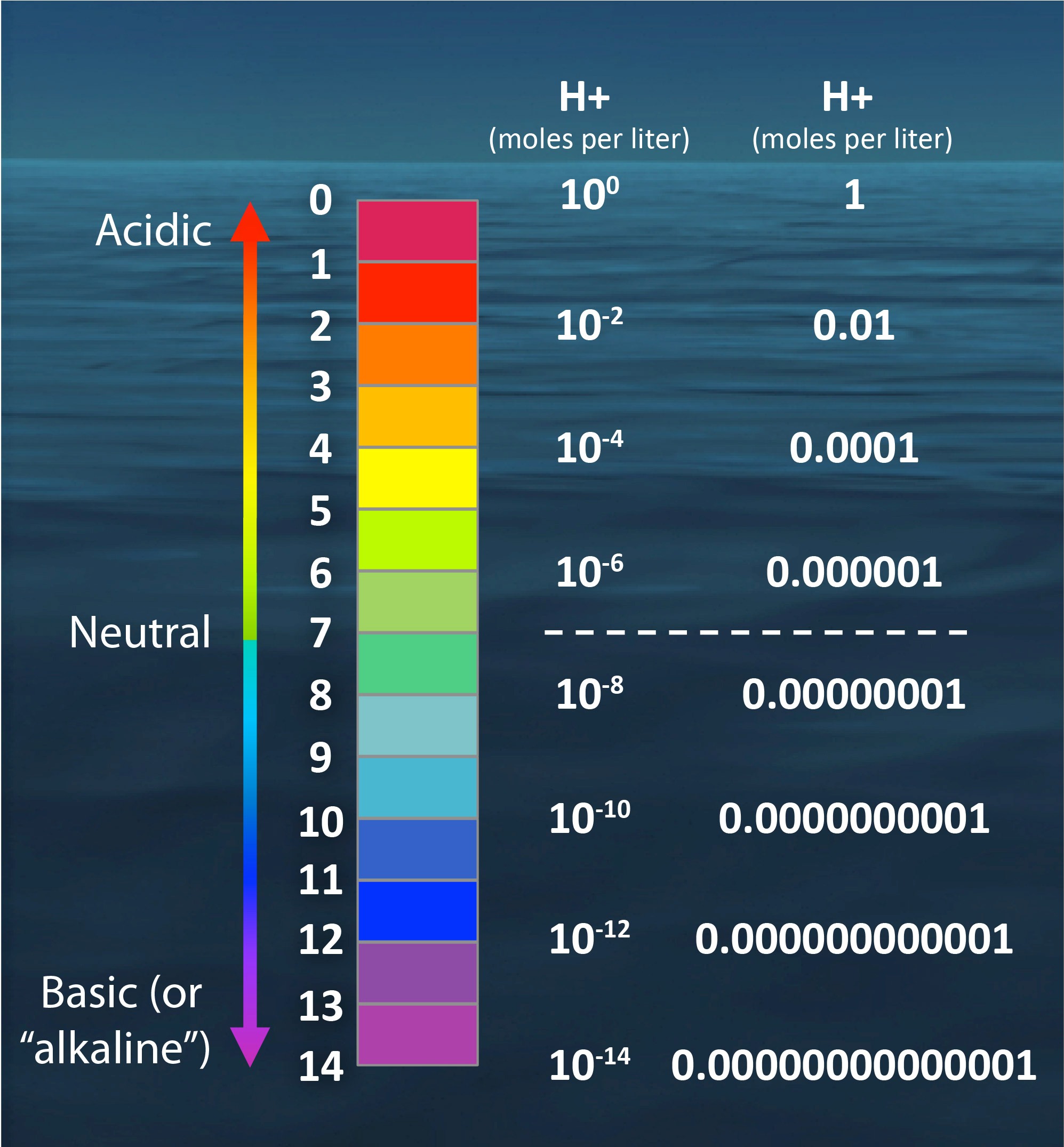





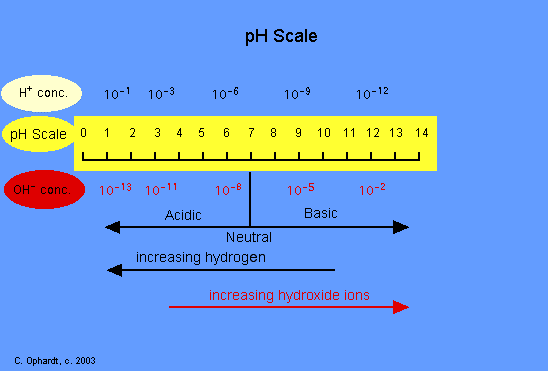
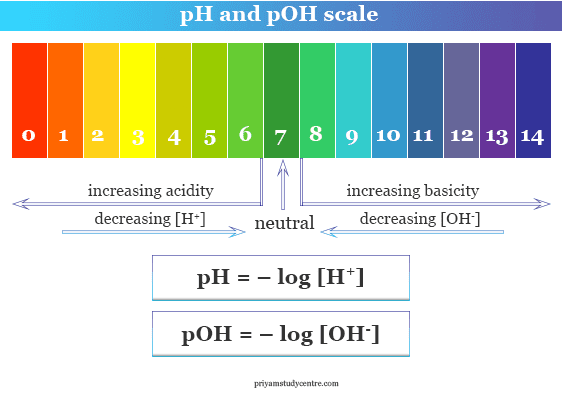

![CHAPTER 19 NOTES: Part III: pH and [H+]. - ppt download CHAPTER 19 NOTES: Part III: pH and [H+]. - ppt download](https://slideplayer.com/slide/15137123/91/images/8/THE+pH+SCALE+The+pH+scale+is+a+logarithmic+scale+from+0+to+14..jpg)

